VISUALIZING ESCHERICHIA COLI SUB-CELLULAR STRUCTURE USING SPARSE DECONVOLUTION SPATIAL LIGHT INTERFERENCE TOMOGRAPHY, PLOS ONE, 7 (6), E38916 (2012)
M. MIR, S. D. BABACAN, M. BEDNARZ, M. N. DO, I. GOLDING, AND G. POPESCU
2012
![]()
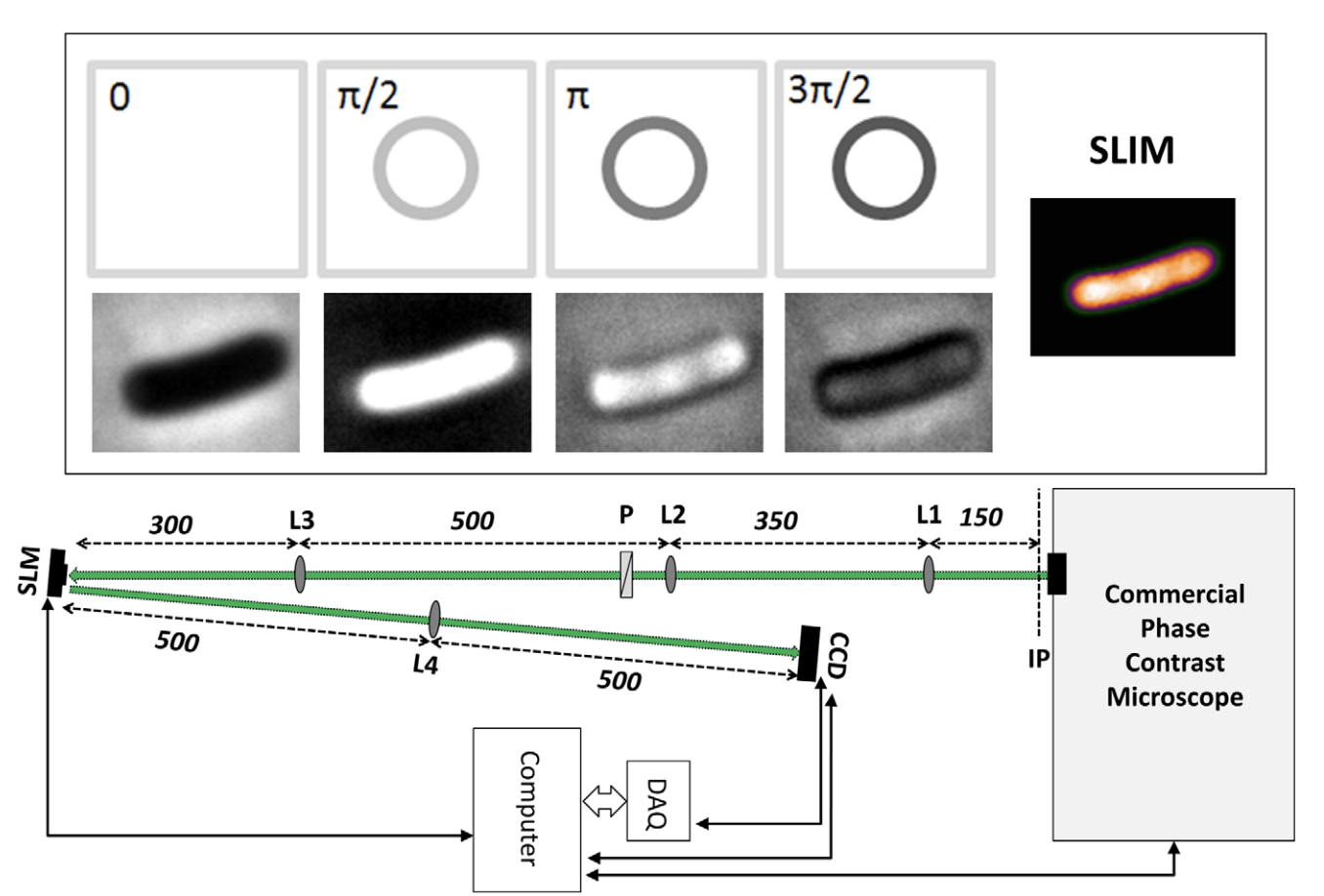
Studying the 3D sub-cellular structure of living cells is essential to our understanding of biological function. However, tomographic imaging of live cells is challenging mainly because they are transparent, i.e., weakly scattering structures. Therefore, this type of imaging has been implemented largely using fluorescence techniques. While confocal fluorescence imaging is a common approach to achieve sectioning, it requires fluorescence probes that are often harmful to the living specimen. On the other hand, by using the intrinsic contrast of the structures it is possible to study living cells in a non-invasive manner. One method that provides high-resolution quantitative information about nanoscale structures is a broadband interferometric technique known as Spatial Light Interference Microscopy (SLIM). In addition to rendering quantitative phaseinformation, when combined with a high numerical aperture objective, SLIM also provides excellent depth sectioning capabilities. However, like in all linear optical systems, SLIM’s resolution is limited by diffraction. Here we present a novel 3D field deconvolution algorithm that exploits the sparsity of phase images and renders images with resolution beyond the diffraction limit. We employ this label-free method, called deconvolution Spatial Light Interference Tomography (dSLIT), to visualize coiled sub-cellular structures in E. coli cells which are most likely the cytoskeletal MreB protein and the division site regulating MinCDE proteins. Previously these structures have only been observed using specialized strains and plasmids and fluorescence techniques. Our results indicate that dSLIT can be employed to study such structures in a practical and non-invasive manner.

