3D-PRINTED PHEMA MATERIALS FOR TOPOGRAPHICAL AND BIOCHEMICAL MODULATION OF DORSAL ROOT GANGLION CELL RESPONSE
Adina Badea,† Joselle M. McCracken,† Emily G. Tillmaand,§ Mikhail E. Kandel,∥ Aaron W. Oraham,† Molly B. Mevis,† Stanislav S. Rubakhin,† Gabriel Popescu,∥ Jonathan V. Sweedler,*,†,§ and Ralph G. Nuzzo*,†,‡
ACS Appl. Mater. Interfaces (2017) 9, 30318−30328 2017
![]()
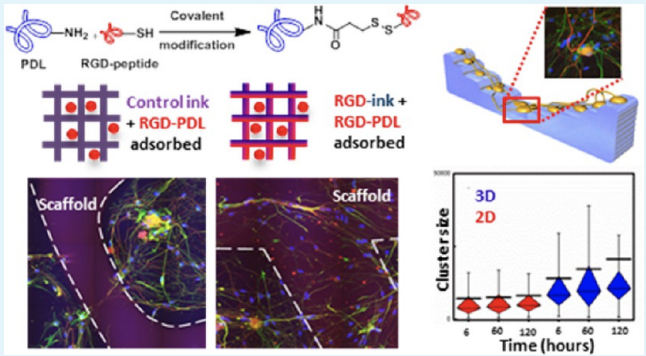
Understanding and controlling the interactions occurring between cells and engineered materials are central challenges toward progress in the development of biomedical devices. In this work, we describe materials for direct ink writing (DIW), an extrusion-based type of 3D printing, that embed a custom synthetic protein (RGD-PDL) within the microfilaments of 3D-hydrogel scaffolds to modify these interactions and differentially direct tissue-level organization of complex cell populations in vitro. The RGD-PDL is synthesized by modifying poly-d-lysine (PDL) to varying extents with peptides containing the integrin-binding motif Arg-Gly-Asp (RGD). Compositional gradients of the RGD-PDL presented by both patterned and thin-film poly(2-hydroxyethyl) methacrylate (pHEMA) substrates allow the patterning of cell-growth compliance in a grayscale form. The surface chemistry-dependent guidance of cell growth on the RGD-PDL-modified pHEMA materials is demonstrated using a model NIH-3T3 fibroblast cell line. The formation of a more complex cellular system—organotypic primary murine dorsal root ganglion (DRG)—in culture is also achieved on these scaffolds, where distinctive forms of cell growth and migration guidance are seen depending on their RGD-PDL content and topography. This experimental platform for the study of physicochemical factors on the formation and the reorganization of organotypic cultures offers useful capabilities for studies in tissue engineering, regenerative medicine, and diagnostics.

