3D‐PRINTED HYDROGEL COMPOSITES FOR PREDICTIVE TEMPORAL (4D) CELLULAR ORGANIZATIONS AND PATTERNED BIOGENIC MINERALIZATION
Joselle M. McCracken, Brittany M. Rauzan, Jacob C. E. Kjellman, Mikhail E. Kandel, Yu Hao Liu, Adina Badea, Lou Ann Miller, Simon A. Rogers, Gabriel Popescu, and Ralph G. Nuzzo*
Adv. Healthcare Mater. (2018) 1800788 2019
![]()
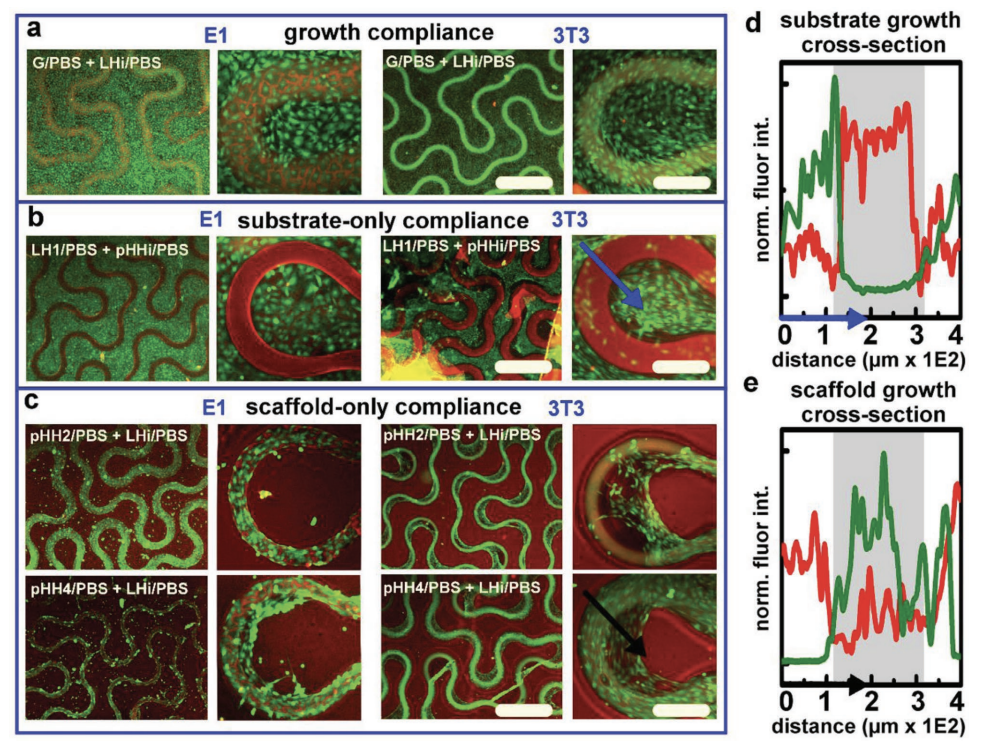
Materials chemistries for hydrogel scaffolds that are capable of programming temporal (4D) attributes of cellular decision‐making in supported 3D microcultures are described. The scaffolds are fabricated using direct‐ink writing (DIW)—a 3D‐printing technique using extrusion to pattern scaffolds at biologically relevant diameters (≤ 100 µm). Herein, DIW is exploited to variously incorporate a rheological nanoclay, Laponite XLG (LAP), into 2‐hydroxyethyl methacrylate (HEMA)‐based hydrogels—printing the LAP–HEMA (LH) composites as functional modifiers within otherwise unmodified 2D and 3D HEMA microstructures. The nanoclay‐modified domains, when tested as thin films, require no activating (e.g., protein) treatments to promote robust growth compliances that direct the spatial attachment of fibroblast (3T3) and preosteoblast (E1) cells, fostering for the latter a capacity to direct long‐term osteodifferentiation. Cell‐to‐gel interfacial morphologies and cellular motility are analyzed with spatial light interference microscopy (SLIM). Through combination of HEMA and LH gels, high‐resolution DIW of a nanocomposite ink (UniH) that translates organizationally dynamic attributes seen with 2D gels into dentition‐mimetic 3D scaffolds is demonstrated. These analyses confirm that the underlying materials chemistry and geometry of hydrogel nanocomposites are capable of directing cellular attachment and temporal development within 3D microcultures—a useful material system for the 4D patterning of hydrogel scaffolds.

