BLOOD TESTING AT THE SINGLE CELL LEVEL USING QUANTITATIVE PHASE AND AMPLITUDE MICROSCOPY, BIOMED. OPT. EXP, 2(12), 2011
M. MIR, K. TANGELLA AND G. POPESCU
2011
![]()
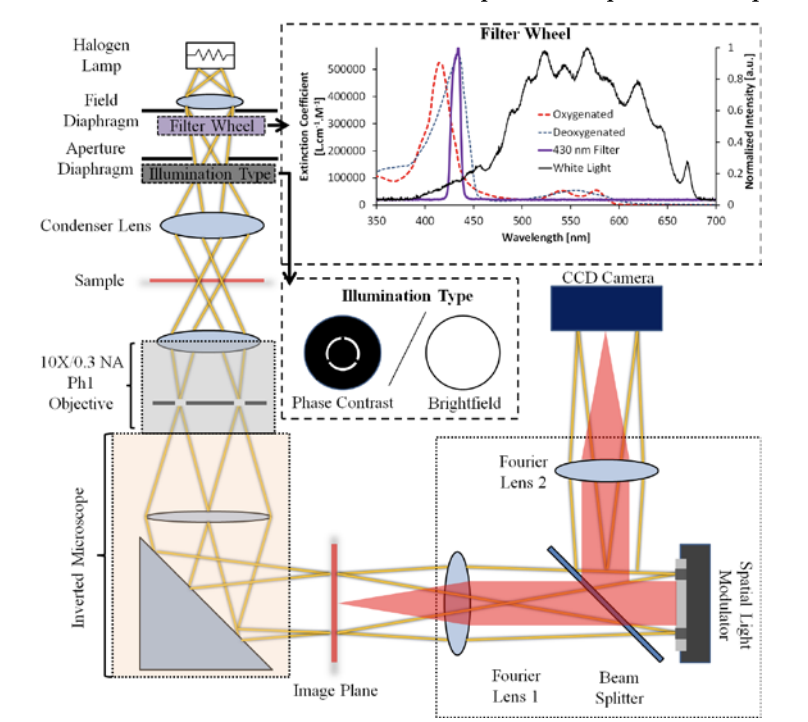
It has recently been shown that quantitative phase imaging methods can provide clinically relevant parameters for red blood cell analysis with unprecedented detail and sensitivity. Since the quantitative phase information is dependent on both the thickness and refractive index, a major limitation to clinical translation has been a simple and practical approach to measure both simultaneously. Here we demonstrate both theoretically and experimentally that, by combining quantitative phase with a single absorption measurement, it is possible to measure both quantities at the single cell level. We validate this approach by comparing our results to those acquired using a clinical blood analyzer. This approach to decouple the thickness and refractive index for red blood cells may be used with any quantitative phase imaging method that can operate in tandem with bright field microscopy at the Soret-band wavelength.
